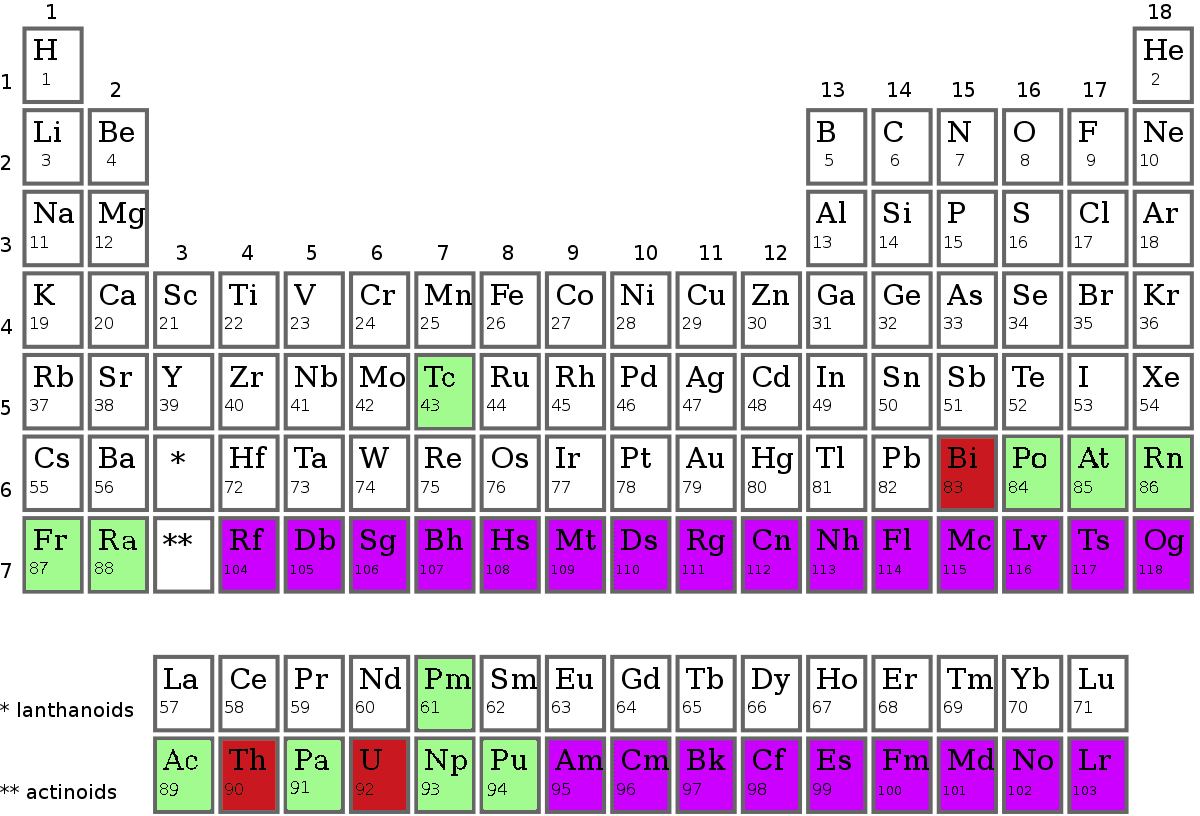
Description
A synthetic element is a known chemical element that does not occur naturally on Earth: it has been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, it is called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table: these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons into the nucleus of an element with an atomic number lower than 95. All known synthetic elements are unstable, but they decay at widely varying rates; the half-lives of their longest-lived isotopes range from microseconds to millions of years.